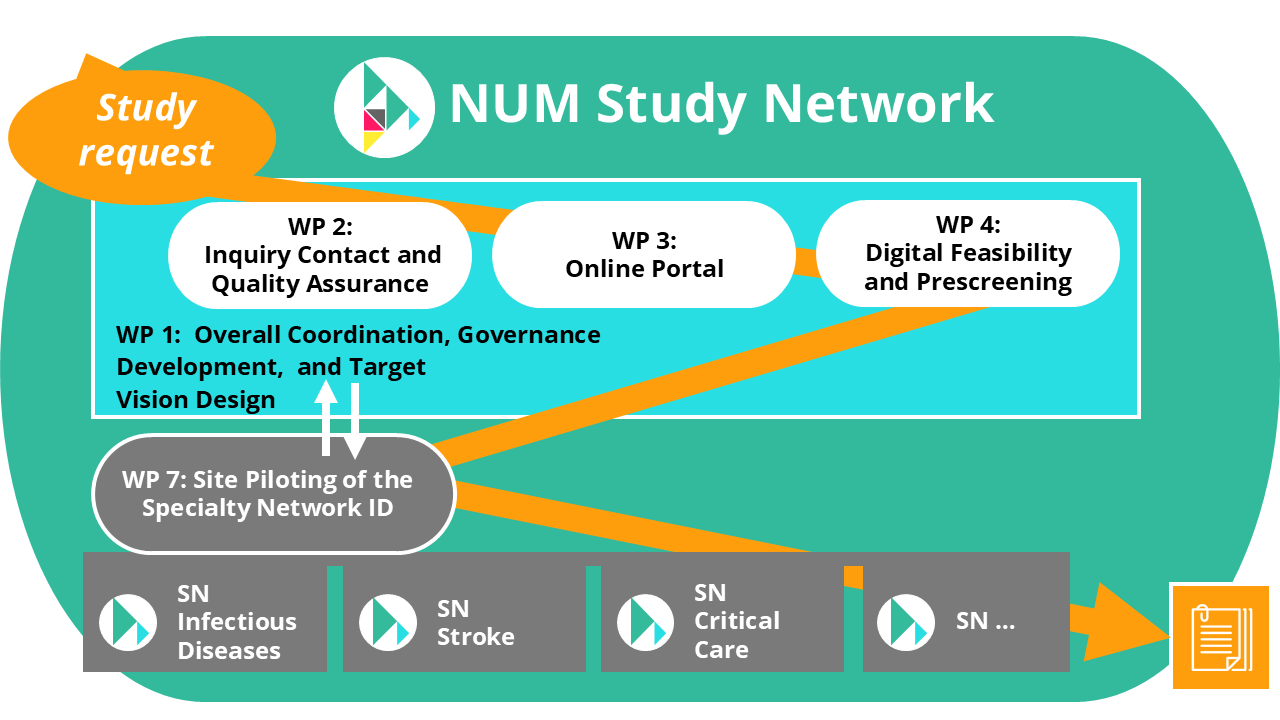
The lifecycle of a clinical study within the NUM Study Network begins with a study request, which enters the system as the initial trigger for the process. This request is routed through various coordinated work packages (WPs) that ensure a structured and high-quality execution from planning to implementation.
WP 2 is responsible for handling inquiry contact and quality assurance, serving as the initial checkpoint for incoming study proposals. Here, feasibility and compatibility with the network’s standards are evaluated.
Following this, the study proceeds through WP 3, where it is integrated into an online portal that facilitates centralized coordination and communication across participating institutions. In parallel, WP 4 handles digital feasibility and prescreening, using digital tools to assess potential study sites and participant eligibility, ensuring that studies are effectively matched with capable locations.
Overarching all of these steps is WP 1, which provides overall coordination, governance development, and the design of a target vision for the network. This foundational work package ensures that every study aligns with the NUM Study Network’s strategic objectives and operational standards.
Once these preparatory phases are complete, the study moves into WP 7, which covers the pilot implementation at selected sites (“Standort-Pilotierung des Fachnetzwerks”). This phase tests and validates the workflows, infrastructure, and readiness of individual centers within the expert networks.
Finally, the study is conducted within one or more of the Specialty Networks — such as Specialty Network Infectious Diseases, Specialty Network Stroke, or Specialty Network Critical Care - depending on the study’s focus area. These thematic networks enable tailored support and expertise, ensuring the study benefits from the highest possible clinical and scientific standards.
Once data is collected and results are analyzed, findings are disseminated through publications in peer-reviewed journals, closing the study lifecycle with scientific impact and public visibility.